How Do You Know Nh3 Is a Base
24.iii: Basicity of Amines
- Page ID
- 36441
Objectives
After completing this section, yous should exist able to
- account for the basicity and nucleophilicity of amines.
- explain why amines are more basic than amides, and improve nucleophiles.
- describe how an amine can be extracted from a mixture that also contains neutral compounds illustrating the reactions which have identify with appropriate equations.
- explain why primary and secondary (but not tertiary) amines may be regarded as very weak acids, and illustrate the synthetic usefulness of the strong bases that can be formed from these weak acids.
Make certain that you can define, and use in context, the fundamental term below.
- amide
The alone pair of electrons on the nitrogen atom of amines makes these compounds not just basic, just also good nucleophiles. Indeed, we accept seen in past chapters that amines react with electrophiles in several polar reactions (see for example the nucleophilic improver of amines in the formation of imines and enamines in Section 19.viii).
The ammonium ions of most elementary aliphatic amines have a pK a of virtually 10 or 11. However, these uncomplicated amines are all more bones (i.e., take a college pK a) than ammonia. Why? Think that, relative to hydrogen, alkyl groups are electron releasing, and that the presence of an electron‑releasing grouping stabilizes ions carrying a positive charge. Thus, the gratis energy difference between an alkylamine and an alkylammonium ion is less than the free energy divergence between ammonia and an ammonium ion; consequently, an alkylamine is more easily protonated than ammonia, and therefore the former has a college pK a than the latter.
Basicity of nitrogen groups
In this department we consider the relative basicity of amines. When evaluating the basicity of a nitrogen-containing organic functional grouping, the fundamental question nosotros demand to ask ourselves is: how reactive (and thus how bones and nucleophilic) is the lone pair on the nitrogen? In other words, how much does that solitary pair desire to break abroad from the nitrogen nucleus and grade a new bail with a hydrogen. The lone pair electrons makes the nitrogen in amines electron dumbo, which is represents by a cherry colour in the electrostatic potential map present beneath left. Amine are basic and easily react with the hydrogen of acids which are electron poor as seen below.
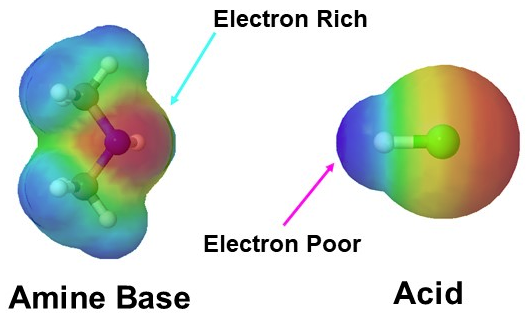
Amines are one of the only neutral functional groups which are considered ground which is a consequence of the presence of the lone pair electrons on the nitrogen. During an acid/base reaction the lone pair electrons assail an acidic hydrogen to grade a N-H bond. This gives the nitrogen in the resulting ammonium salt four single bonds and a positive accuse.
Amines react with water to plant an equilibrium where a proton is transferred to the amine to produce an ammonium salt and the hydroxide ion, equally shown in the following full general equation:
\[RNH2_{(aq)}+H_2O_{(l)} \rightleftharpoons RNH3^+_{(aq)}+OH^−_{(aq)} \label{16.five.4}\]
The equilibrium abiding for this reaction is the base ionization constant (1000b), also called the base dissociation constant:
\[K_b=\dfrac{[RNH3^+][OH^−]}{[NH2]} \label{16.five.5}\]
pKb = -log Kb
Simply as the acid forcefulness of a carboxylic acid tin can be measured by defining an acidity abiding Thousanda (Section 2-8), the base strength of an amine can be measured by defining an analogous basicity constant Thoub. The larger the value of Thoub and the smaller the value of pKb, the more favorable the proton-transfer equilibrium and the stronger the base.
However, Kb values are oftentimes not used to discuss relative basicity of amines. Information technology is mutual to compare basicity's of amines past using the Ka's of their conjugate acids, which is the respective ammonium ion. Fortunately, the Ka and Kb values for amines are straight related.
Consider the reactions for a conjugate acid-base pair, RNH3 + − RNH2:
\[\ce{RNH3+}(aq)+\ce{H2O}(fifty)⇌\ce{RNH2}(aq)+\ce{H3O+}(aq) \hspace{20px} K_\ce{a}=\ce{\dfrac{[RNH2][H3O]}{[RNH3+]}}\]
\[\ce{RNH2}(aq)+\ce{H2o}(50)⇌\ce{RNH3+}(aq)+\ce{OH-}(aq) \hspace{20px} K_\ce{b}=\ce{\dfrac{[RNH3+][OH-]}{[RNH2]}}\]
Adding these 2 chemic equations together yields the equation for the autoionization for water:
\[\abolish{\ce{RNH3+}(aq)}+\ce{H2o}(l)+\cancel{\ce{RNH2}(aq)}+\ce{H2O}(fifty)⇌\ce{H3O+}(aq)+\abolish{\ce{RNH2}(aq)}+\ce{OH-}(aq)+\cancel{\ce{RNH3+}(aq)}\]
\[\ce{2H2O}(l)⇌\ce{H3O+}(aq)+\ce{OH-}(aq)\]
Given that the K expression for a chemical equation formed from adding two or more other equations is the mathematical product of the input equations' M constants.
Ka Ten Kb = {ii H2O} / (H3O+}{OH-} = Kw
\[K_\ce{a}=\dfrac{K_\ce{w}}{K_\ce{b}}\]
pKa + pKb =14
Thus if the Thousanda for an ammonium ion is know the Kb for the corresponding amine can be calculated using the equation Chiliadb = Kw / Ka. This relationship shows that every bit an ammonium ion becomes more acidic (Ka increases / pKa decreases) the correspond base becomes weaker (Kb decreases / pKb increases)
Weaker Base of operations = Larger Chiliada and Smaller pKa of the Ammonium ion
Stronger Base of operations = Smaller Ka and Larger pKa of the Ammonium ion
Similar ammonia, most amines are Brønsted-Lowry and Lewis bases, but their base of operations forcefulness can exist changed enormously past substituents. Most unproblematic alkyl amines have pKa's in the range nine.5 to 11.0, and their aqueous solutions are basic (have a pH of 11 to 12, depending on concentration).
Effluvious herterocyclic amines (such equally pyrimidine, pyridine, imidazole, pyrrole) are significantly weaker bases as a consequence of iii factors. The first of these is the hybridization of the nitrogen. In each case the heterocyclic nitrogen is sp2 hybridized. The increasing south-character brings information technology closer to the nitrogen nucleus, reducing its tendency to bond to a proton compared to sp3 hybridized nitrogens. The very low basicity of pyrrole reflects the exceptional delocalization of the nitrogen electron pair associated with its incorporation in an aromatic ring. Imidazole (pKa = vi.95) is over a 1000000 times more than basic than pyrrole because the sp2 nitrogen that is part of one double bail is structurally like to pyridine, and has a comparable basicity.
Basicity of mutual amines (pKa of the conjugate ammonium ions)
Anterior Effects in Nitrogen Basicity
Alkyl groups donate electrons to the more electronegative nitrogen. The inductive effect makes the electron density on the alkylamine's nitrogen greater than the nitrogen of ammonia. The modest corporeality of extra negative charge built up on the nitrogen cantlet makes the lone pair fifty-fifty more attractive towards hydrogen ions. Correspondingly, primary, secondary, and tertiary alkyl amines are more bones than ammonia.
| Compound | pKa |
| NH3 | 9.3 |
| CH3NH2 | x.66 |
| (CH3)2NH | x.74 |
| (CHiii)3N | 9.81 |
Comparison the Basicity of Alkylamines to Amides
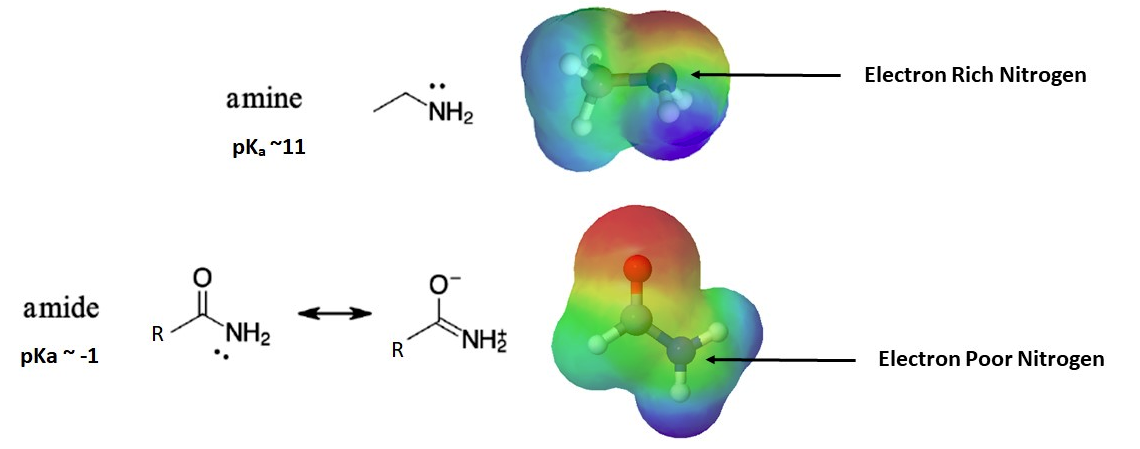
The nitrogen atom is strongly basic when it is in an amine, but not significantly bones when information technology is office of an amide grouping. While the electron lone pair of an amine nitrogen is localized in 1 identify, the lone pair on an amide nitrogen is delocalized by resonance. The electron density – in the form of a lone pair – is stabilized by resonance delocalization, even though at that place is not a negative charge involved. Hither's another way to call back about it: the lone pair on an amide nitrogen is not equally available for bonding with a proton – these two electrons are too stable being part of the delocalized pi-bonding system. The electrostatic potential map shows the outcome of resonance on the basicity of an amide. The map shows that the electron density, shown in red, is almost completely shifted towards the oxygen. This profoundly decreases the basicity of the alone pair electrons on the nitrogen in an amide.
Amine Extraction in the Laboratory
Extraction is frequently employed in organic chemistry to purify compounds. Liquid-liquid extractions take reward of the divergence in solubility of a substance in two immiscible liquids (east.g. ether and water). The ii immiscible liquids used in an extraction process are (ane) the solvent in which the solids are dissolved, and (2) the extracting solvent. The 2 immiscible liquids are and so easily separated using a separatory funnel. For amines one can take advantage of their basicity by forming the protonated salt (RNH2 +Cl−), which is soluble in water. The salt will extract into the aqueous phase leaving backside neutral compounds in the non-aqueous phase. The aqueous layer is then treated with a base (NaOH) to regenerate the amine and NaCl. A second extraction-separation is then done to isolate the amine in the not-aqueous layer and leave behind NaCl in the aqueous layer.
Important Reagent Bases
The significance of all these acrid-base of operations relationships to practical organic chemistry lies in the need for organic bases of varying forcefulness, as reagents tailored to the requirements of specific reactions. The mutual base of operations sodium hydroxide is not soluble in many organic solvents, and is therefore not widely used as a reagent in organic reactions. Most base reagents are alkoxide salts, amines or amide salts. Since alcohols are much stronger acids than amines, their conjugate bases are weaker than amine bases, and fill the gap in base force between amines and amide salts.
| Base Name | Pyridine | Triethyl Amine | Hünig's Base | Barton's Base | Potassium t-Butoxide | Sodium HMDS | LDA |
|---|---|---|---|---|---|---|---|
| Formula | | (C2Hfive)3Due north | | | (CHiii)3CO(–) K(+) | [(CH3)iiiSi]iiN(–) Na(+) | [(CH3)iiCH]2N(–) Li(+) |
| pKa of conjugate acid | 5.3 | 10.seven | eleven.4 | xiv | 19 | 26 | 35.7 |
Basicity of common amines (pKa of the conjugate ammonium ions)
Pyridine is commonly used as an acid scavenger in reactions that produce mineral acid co-products. Its basicity and nucleophilicity may be modified by steric hindrance, as in the example of two,six-dimethylpyridine (pKa=6.7), or resonance stabilization, equally in the case of 4-dimethylaminopyridine (pKa=9.7). Hünig'south base is relatively not-nucleophilic (due to steric hindrance), and is often used every bit the base of operations in E2 elimination reactions conducted in not-polar solvents. Barton'southward base is a strong, poorly-nucleophilic, neutral base that serves in cases where electrophilic commutation of other amine bases is a problem. The alkoxides are stronger bases that are often used in the corresponding booze as solvent, or for greater reactivity in DMSO. Finally, the two amide bases run into widespread utilize in generating enolate bases from carbonyl compounds and other weak carbon acids.
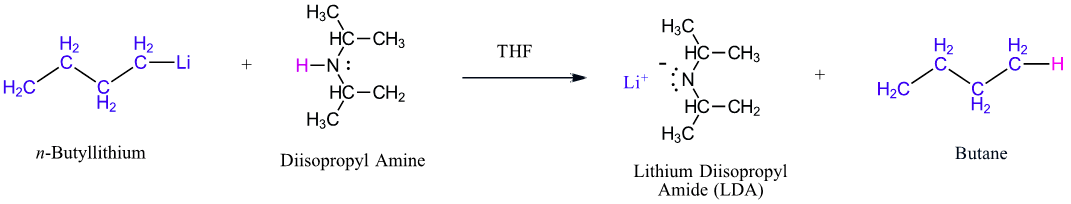
In addition to interim every bit a base, 1o and 2o amines can human activity equally very weak acids. Their N-H proton can be removed if they are reacted with a strong enough base. An example is the formation of lithium diisopropylamide (LDA, LiN[CH(CH3)two]ii) by reacting n-butyllithium with diisopropylamine (pKa 36) (Section 22-5). LDA is a very strong base and is commonly used to create enolate ions by deprotonating an alpha-hydrogen from carbonyl compounds (Section 22-7).
Exercises
Q24.iii.one
Select the more basic amine from each of the following pairs of compounds.
(a)
(b)
(c)
Q24.three.ii
The 4-methylbenzylammonium ion has a pKa of 9.51, and the butylammonium ion has a pKa of ten.59. Which is more than basic? What's the pKb for each compound?
Solutions
S24.3.1
(a)
(b)
(c)
S24.three.two
The butylammonium is more than bones. The pKb for butylammonium is 3.41, the pKb for iv-methylbenzylammonium is 4.49
Source: https://chem.libretexts.org/Bookshelves/Organic_Chemistry/Organic_Chemistry_(McMurry)/24%3A_Amines_and_Heterocycles/24.03%3A_Basicity_of_Amines
0 Response to "How Do You Know Nh3 Is a Base"
Post a Comment